Highlights from CITY and MILLENNIAL studies of CGM in teens with T1D
Two recently published randomized control trials (RCTs) show the value of Dexcom Continuous Glucose Monitoring (CGM) Systems for teens and young adults with Type 1 diabetes (T1D). Both trials’ findings are highly meaningful as T1D registries have shown that A1C levels are highest in adolescents and young adults.1 Historically, CGM adoption1 and usage rates2 in this population have been low.
The CITY Study
A randomized control trial recently published in the Journal of the American Medical Association sought to determine the effect of a Dexcom G5 Mobile Continuous Glucose Monitoring (CGM) System on glycemic control in 153 adolescents and young adults (ages 14-24) with T1D compared to usual care with fingerstick testing.3 The cohort chosen for this study was diverse (38% non-white) and 40% of the participants were on public insurance. The primary endpoint was a change in A1C from baseline to 26 weeks. The authors reported the following results:
- A significant mean reduction of -0.4 percentage points in A1C in the CGM group (from 8.9% to 8.5%; P=0.01) at 26 weeks; no change was observed in the control group.
- Participants randomized to Dexcom CGM were more than twice as likely to achieve an A1C reduction of ≥0.5% than those who monitored their glucose with traditional fingerstick testing (P = 0.005).
- Participants randomized to Dexcom CGM increased the average time they spent in range (TIR, 3.9-10.0 mmol/L) by more than 1.7 hours each day (P <0.001) compared to those who monitored their glucose with traditional fingerstick testing.
- More than two-thirds (68%) of participants used CGM at least five days per week after six months.
- Half (50%) of participants chose to share their CGM data with another person.
The MILLENNIAL Study
Diabetes Care recently published a randomized crossover trial from the UK using the Dexcom G6 Continuous Glucose Monitoring (CGM) System.4 This study included a diverse cohort of 32 young adults (ages 16-24) studied over 20 weeks. Comparing CGM to self-monitoring of blood glucose (SMBG), the primary endpoint was time in range (TIR). Below are some of the published outcomes:
- Mean TIR was 11.1 percentage points higher during Dexcom G6 use compared to usual care with SMBG (P <0.001).
- This correlates to a mean increase in TIR of ~2.7 hours daily.
- Mean time spent in hyperglycemia was significantly lower during Dexcom G6 use compared to usual care with SMBG (P < 0.001).
- Times spent in hypoglycemia (<3.9 mmol/L and <3.0 mmol/L) were low and comparable during both study periods.
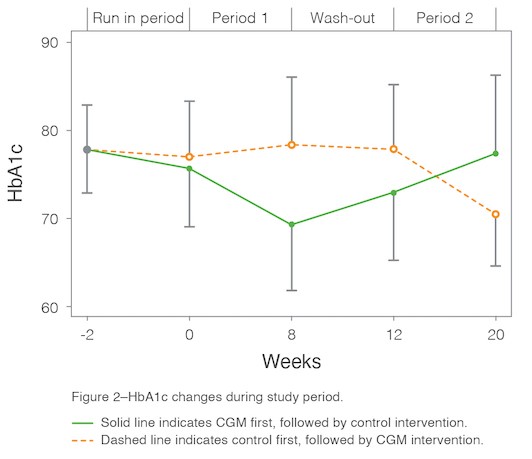
- The mean difference in A1C between the control period and Dexcom G6 wear period was significant and clinically relevant at -0.76 percentage points (P <0.001). Participants using CGM returned to baseline A1C when they managed their diabetes with SMBG after the crossover period.
- Participants reported a higher total mean score on the Glucose Monitoring Satisfaction Survey when using Dexcom G6 compared to usual care with SMBG (P = 0.011).
- Participants wore their CGM 84% of the time, suggesting that they were finding value in their Dexcom G6.
The MILLENNIAL randomized crossover trial demonstrates how CGM lowers A1C in young adults
1 Foster NC, et al. Diabetes Technol Ther. 2019;21(2):66-72.
2 JDRF CGM Study Group. N Engl J Med. 2008;359(14):1464-1476.
3 Laffel LM, et al. JAMA.2020;323(23):2388–2396.
4 Thabit H, et al. Diabetes Care 2020;43(10):2537-43.
LBL022292 Rev001
